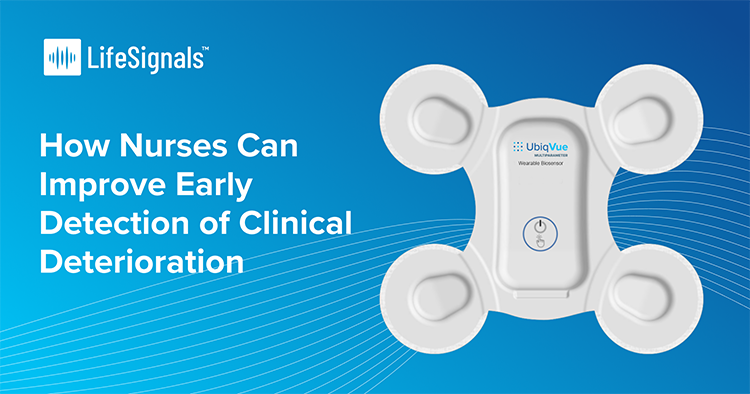
LifeSignals Showcases UbiqVue Multiparameter System at Arab Health 2025
Milpitas, California, USA. January 10, 2025: LifeSignals, Inc. today announced that the UbiqVueTM Multiparameter System will be presented at Arab Health 2025, 27th – 30th January in Dubai, UAE. The end-to-end wireless system, with FDA 510(k) clearance and MDR Certification, represents a significant milestone in advancing continuous patient monitoring for population health management. UbiqVue Multiparameter …
LifeSignals Showcases UbiqVue Multiparameter System at Arab Health 2025 Read More »